Liquid mercury, in schools, poses three major problems:
- It is extremely toxic,
- It has a high vapor pressure, so you can be poisoned by invisible mercury vapor leaving any exposed surface of liquid mercury, and
- Playing with liquid mercury is a lot of fun.
These are compelling reasons to leave use of mercury to those at the college level, or beyond. In the opinion of this science teacher, use of liquid mercury in science classes, up through high school chemistry, inside or outside thermometers, is a bad idea. If the bulb at the bottom of a thermometer, as well as the colored stripe, looks silvery, as in the picture below (found on Wikipedia), then that silvery liquid is mercury, and that thermometer should not be used in labs for high school, let alone with younger children. Your local poison control center can help you find the proper thing to do with mercury in your area; it should definitely not just be thrown away, for we do not need this serious environmental toxin in landfills, where it will eventually reach, and poison, water. Red-stripe thermometers without any silvery line, on the other hand, are far safer, although broken glass can still cause injury.

I turned ten years old in 1978, and, by that time, I had already spent many hours playing (unsupervised) with liquid mercury, pouring it hand-to-hand, etc., so I know exactly how irresistible a “plaything” mercury can be, to children. Luck was on my side, and I suffered no ill effects, but I can state from experience that children should not be tempted with highly-toxic “mercury as a toy,” for it’s not a toy at all. Mercury spills require special “hazmat” training to clean up safely; anyone encountering such a spill who does not have such training should simply notify the proper authorities. In the USA, this means evacuating the area immediately, and then calling 911 — from far enough away to keep the caller from breathing invisible mercury vapor.
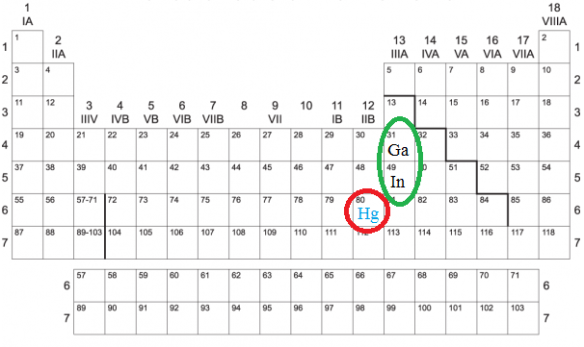
Fortunately, there is a safe alternative which can give students a chance to experiment with a room-temperature metal: an alloy of three parts gallium to one part indium, by mass. Gallium’s melting point is between normal human body temperature and room temperature, so it can literally melt in your hand (although a hot plate is faster). Indium, on the other hand, has a melting point of 156.6°C. For this reason, I will not buy a hot plate unless it can reach higher that that temperature. (Note: use appropriate caution and safety equipment, such as goggles and insulated gloves, with hot plates, and the things heated with them, to avoid burns.)
Once both elements are massed, in the proportions given above, they can then be melted in the same container. When they melt and mix together, they form an alloy which remains liquid at room temperature.
Some might wonder how mixing two elements can create an alloy with a melting point below the melting points of either of the two ingredients, and the key to that puzzle is related to atomic size. Solids have atoms which vibrate back and forth, but don’t move around each other. In liquids, the atoms are more disordered (and faster), and easily slip around each other. In solid, room-temperature gallium, all the atoms are of one size, helping the solid stay solid. Warm it a little, and it melts. With pure indium, this applies, also, but you have to heat it up a lot more to get it to melt. If the two metals are melted and thoroughly mixed, though, and then frozen (a normal freezer is cold enough), the fact that the atoms are of different sizes (indium atoms are larger than gallium atoms) means the atoms will be in a relatively disordered state, compared to single-element solids. In liquids, atoms are even more disordered (that is, they possess more entropy). Therefore, a frozen gallium/indium alloy, with two sizes of atoms, is already closer to a disordered, liquid state, in terms of entropy, than pure, solid gallium or indium at the same temperature. This is why the gallium-indium mixture has a melting point below either individual element — it requires a lower temperature to get the individual atoms to flow past each other, if they are already different atoms, with different sizes.
Those who have experience with actual liquid mercury will notice some important differences between it and this gallium-indium alloy, although both do appear to be silver-colored liquids. (This is why mercury is sometimes called “quicksilver.”) For one thing, their densities are different. A quarter, made of copper and nickel, will float on liquid mercury, for the quarter’s density is less than that of mercury. However, a quarter will sink in liquid 3:1 gallium-indium alloy. To float a metal on this alloy, one would need to use a less-dense metal, such as aluminum or magnesium, both of which sink in water, but float in liquid Ga/In alloy.
Other differences include surface tension; mercury’s is very high, causing small amounts of it on a floor to form little liquid balls which are difficult (and dangerous) to recapture. Gallium-indium alloy, by contrast, has much less surface tension. As a result, unlike mercury, this alloy does not “ball up,” and it will wet glass — and doing that turns the other side of the glass into a mirror. Actual mercury will not wet glass.
The most important differences, of course, is that indium and gallium are far less toxic than mercury, and that this alloy of those two elements has a much lower vapor pressure than that of mercury. Gallium and indium are not completely non-toxic, though. Neither indium nor gallium should be consumed, of course, and standard laboratory safety equipment, such as goggles and gloves, should be worn when doing laboratory experiments with these two elements.